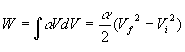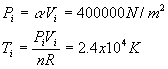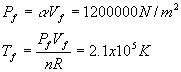|
|
|
|
Real Exam Au00 + Solutions |
Student Peformance! |
|
1. 20 points. A room of volume V = 30.0 m3 contains air molecules at temperature T1 = 30 oC. If the temperature of the room is decreased to T2 = 16 oC, how many moles of air have entered the room? Assume the air pressure of the room is maintained at a constant pressure P = 1.00 x 10 5 N/m2. |
|
|
Solution Outline: See Quiz 1. In that problem, the gas leaves the room as the temperature increases at constant pressure! This problem is the same, except that here the gas enters the room as the temperature decreases at constant pressure! |
|
|
2. 20 points. A 1.50-kg iron (FE) horseshoe initially at exactly 600oC is dropped into a bucket containing 20.0 kg of water (w) . The bucket is made of aluminum (al) and has mass 3.00 kg. The water and bucket are initially at 25.0 oC.
|
|
|
Solution Outline: See Quiz 2. In that problem, you pour hot water into the water and the container. . In this problem, you drop a hot piece of metal into the water and container. |
|
|
3. 26 points. 2 moles of a sample of gas is expanded from Vi = 1.00 m3 to Vf = 3.00 m3. The pressure obeys the following relationship as a function of the volume V: where a = 4.00 atm/m3 Note: R = 8.315 J/mol× K and 1 atm = 1.013x105 N/m2 . a. (6) Derive the work W done by the gas using symbols. Use only symbols for this part. b. (2) What is the numerical value( in Joules) of the work W after you plug in the values given in the problem? c. (3)What is the initial temperature of the gas? d. (3)What is the final temperature of the gas? e. (6)What is the change in internal energy D U of the gas? Assume a diatomic gas in which the molecules rotate and vibrate. f. (6)How much heat Q is transferred to the gas?
|
|
|
Solution Outline: See the sample exam. (a) (e) delta U = (7/2)nR(Tf - Ti) = 1.1x107 J |
|
|
4. ( 8 points) Extra Credit. A 1.00-kg horseshoe (Fe) is taken from a furnace at 900oC and dropped into a bucket containing 4.00 kg of water (w) at 10.0oC. Ignore the specific heat of the bucket. Assume no energy is lost by heat to the surroundings. (a) (6) What is the total entropy change of the system (horseshoe + water)? Use Cw = 4186 J/kg oC and CFe = 448 J/kgoC . (b) (2) Is the process irreversible? Prove you answer.
|
|
|
Solution Outline: See Quiz 4. You did that problem!! This problem is similar. (a) MwCw(Tf - 10) = Mfe*Cfe*(900 - Tf). From this, we get Tf = 33 degrees C (b) Irreversible because delta S > 0. If the process is irreversible, the entropy of an isolated system always increases. Thus, the change delta S > 0. This is the second law of thermodynamics!! |
|